10 Aug FDA grants priority review for Stratatech tissue to treat burns
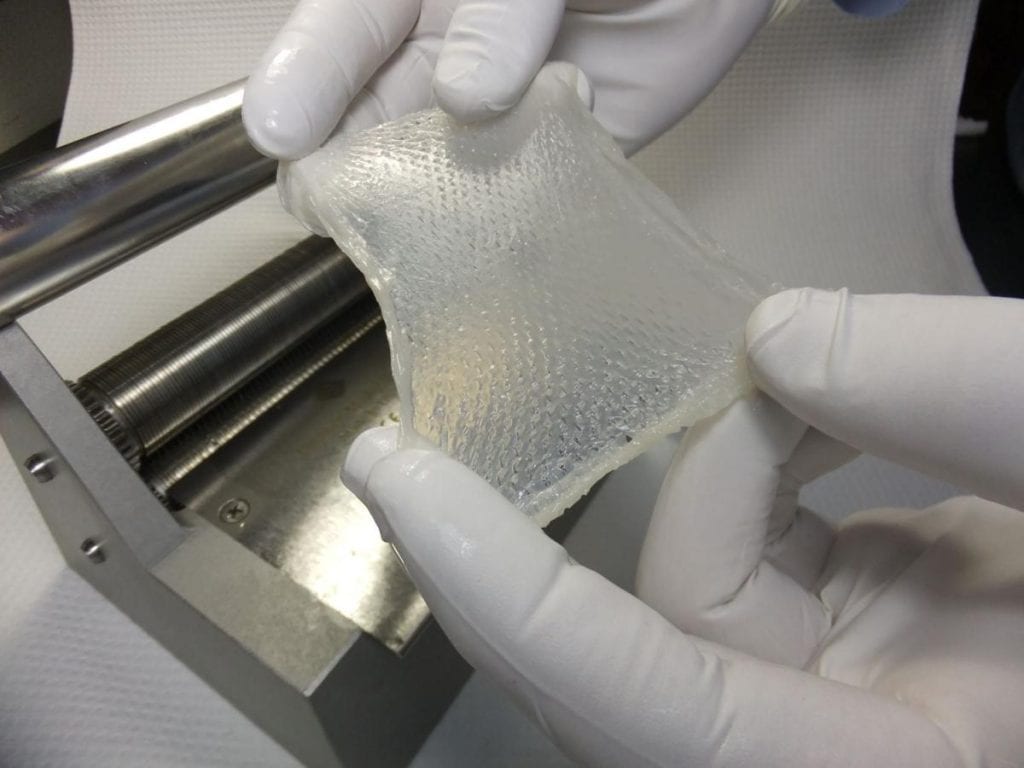
StrataGraft, skin tissue to treat burn wounds that was developed in Madison, will get a stepped-up review, the FDA says. PHOTO: STRATATECH/MALLINCKRODT
The Food and Drug Administration has granted priority review to an application for StrataGraft, a regenerative tissue developed by a Madison company as an alternative to skin grafts in treating burns.
Stratatech submitted its biologics license application to the FDA in June. The company, started in 2000, is based on research from the UW-Madison lab of pathologist Lynn Allen-Hoffmann.
Stratatech, which has about 100 employees, is located at University Research Park on Madison’s West Side.
The goal for StrataGraft is to prevent or reduce the need to use painful autografts, or skin grafts from a patient’s own body, to cover up burn wounds and help them heal.



