12 Jun Stratatech starts latest study of skin-like tissue in burn patients
By Jeff Buchanan, Xconomy
Stratatech said this week that the first patient has been enrolled in a Phase 3 clinical trial of the cell-based regenerative skin tissue the company is developing.
The aim of the study is to assess the safety and efficacy of StrataGraft, the company’s flagship skin replacement product, at coaxing the bodies of patients with burn wounds into regenerating their own skin.
Madison, WI-based Stratatech is owned by the U.K. pharmaceuticals giant Mallinckrodt (NYSE: MNK), which acquired Stratatech a year ago for $76 million in cash, plus potential future milestone payments.
Stratatech estimates it will enroll 70 patients in the study, which is estimated to be completed in late 2019, according to trial information posted on ClinicalTrials.gov.
“We are very excited to further study StrataGraft to learn how it could meet the needs of patients,” said lead investigator James Holmes IV in a prepared statement. Holmes is a surgeon who directs the burn center at Wake Forest Baptist Medical Center, one of the clinical trial sites.
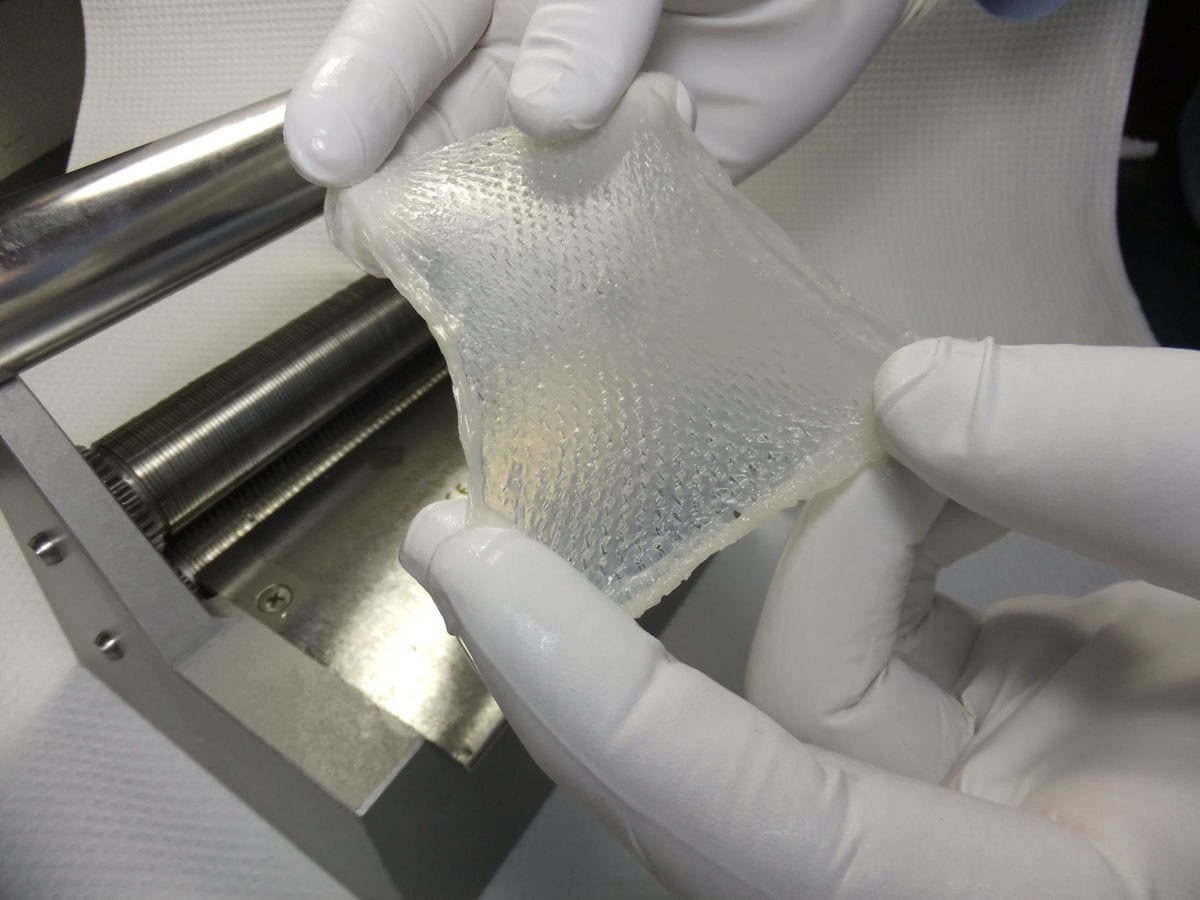
StrataGraft is a sheet of living tissue made from a type of human keratinocyte progenitor cells that develop into skin. According to Stratatech, StrataGraft tissue can be sutured, stapled, or put into place using an adhesive. Read more …



