28 Oct Stratatech to expand tests of its skin tissue on pediatric burn patients
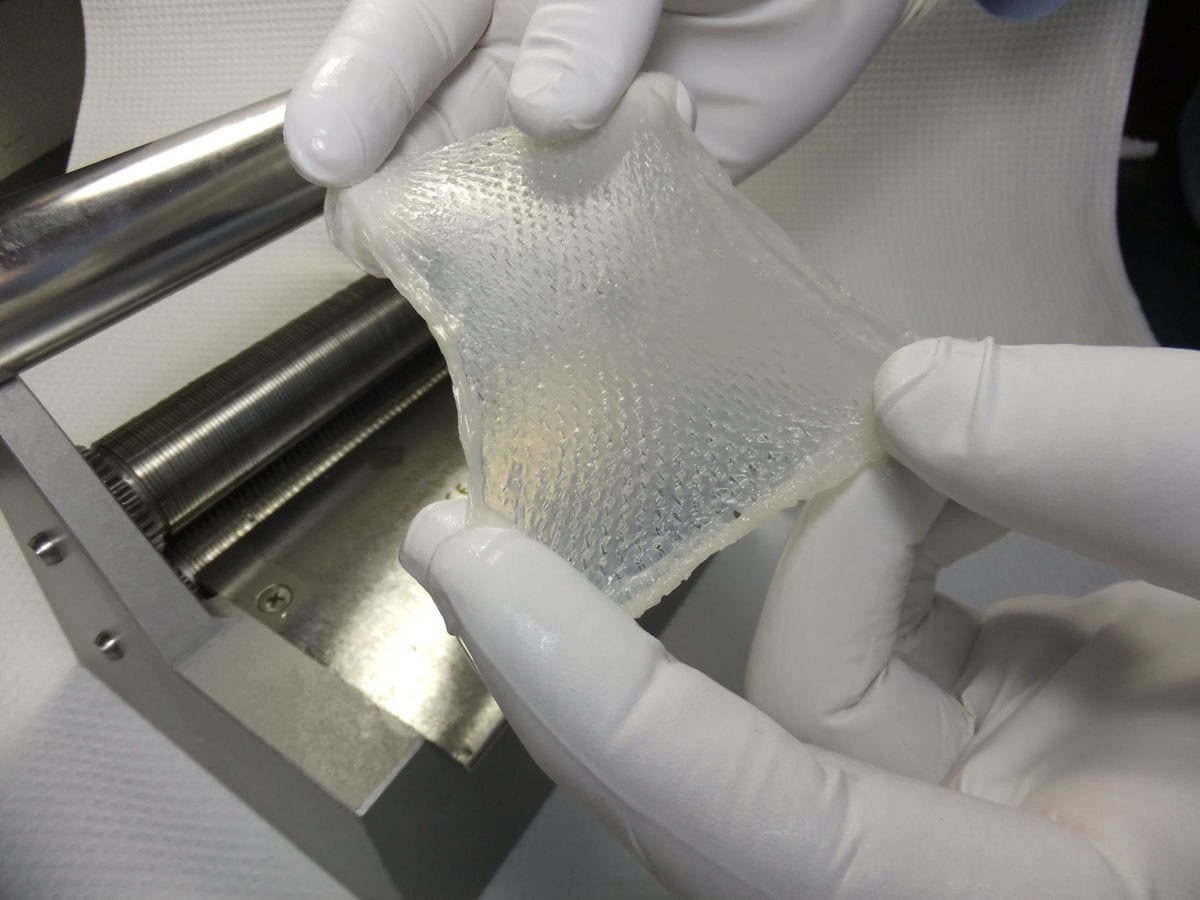
Stratatech is starting a test of its StrataGraft skin tissue to see how well it works on children who have suffered serious burns.
It’s the first time the Madison company — a subsidiary of British-based Mallinckrodt Pharmaceuticals — will study the use of its engineered skin tissue on pediatric burn patients; up to now, only adult patients have gone through the treatment in clinical trials.
In a continuing partnership with the U.S. Department of Health and Human Services, Stratatech is getting another $26 million from Project BioShield, a program of the Biomedical Advanced Research and Development Authority (BARDA).



